

Medical Device companies need to consider design reviews, not as checking a box, but adding real value to help design a medical device that meets user needs and provide a successful design. Design Reviews a regulatory requirement to meet ISO 13485:2016 as well as FDA. They are also a way to ensure and document the design and development process is on track and meeting planned expectations. Design Reviews are conducted throughout the project as shown in the diagram below. This article is to offer some tips on how to conduct productive and successful design reviews.
Before we get into the details on design reviews let?s take a look at the Regulatory requirements:
Design and Development Review requirements to meet ISO 13485:2016 Section 7.3.5 and FDA CFR 21 Part 820.30(e):
ISO 13485:2016: Section 7.3.5 Design and development review, states the following:
At suitable stages, systematic reviews of design and development shall be performed in accordance with planned and documented arrangements to:
a) evaluate the ability of the results of design and development to meet requirements.
b) identify and propose necessary actions.
Participants in such reviews shall include representatives of functions concerned with the design and development stage being reviewed as well as other specialist personnel.
Records of the results of the reviews and any necessary actions shall be maintained and include the identification of the design under review, the participants involved and the date of the review (see 4.2.5)
FDA CFR 21 Part 820.30(e), states the following:
Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device?s design development. The procedures shall ensure that participants at each design review include representatives of all functions concerned with the design stage being reviewed and an individual(s) who does not have direct responsibility for the design stage being reviewed, as well as any specialists needed. The results of a design review, including identification of the design, the date, and the individual(s) performing the review, shall be documented in the design history file (the DHF).
As you can see the ISO 13485 and FDA requirements are very similar with the only real addition under the FDA being the requirement for an ?independent reviewer? to participate in the design review.
Introduction
FDA 820.3(h) defines design review as follows:
Design review means a documented, comprehensive, systematic examination of a design to evaluate the adequacy of the design requirements, to evaluate the capability of the design to meet these requirements, and to identify problems.
Here is some additional guidance offered by the FDA on design reviews:
In general, formal design reviews are intended to :
- provide a systematic assessment of design results, including the device design and the associated designs for production and support processes.
- provide feedback to designers on existing or emerging problems.
- assess project progress; and/or;
- provide confirmation that the project is ready to move on to the next stage of development.
Many types of reviews occur during the course of developing a product. Reviews may have both an internal and external focus. The internal focus is on the feasibility of the design and the producibility of the design with respect to manufacturing and support capabilities. The external focus is on the user requirements; that is, the device design is viewed from the perspective of the user.
For more on FDA guidance on design reviews and the overall design and development process check out the link below for a copy of FDA Design Control Guidance for Medical Device Manufacturers.
click for FDA Design Control Guidance Document
Tips for Successful Design Reviews
Successful design reviews that will contribute to the design and development process and not just tick-the-box will depend on how well you plan, conduct, and follow-up on your reviews. So here are my recommendations for design reviews based on my experience leading design projects for Design Engineering, and later as a Quality and Regulatory leader.
Planning:
So as shown in this article and under the ISO and FDA requirements for design review planning, ISO requires ?at suitable stages, systematic reviews of design and development shall be performed in accordance with planned and documented arrangements?, and the FDA requires, ?formal documented reviews of the design results are planned and conducted at appropriate stages of the device?s design development?.
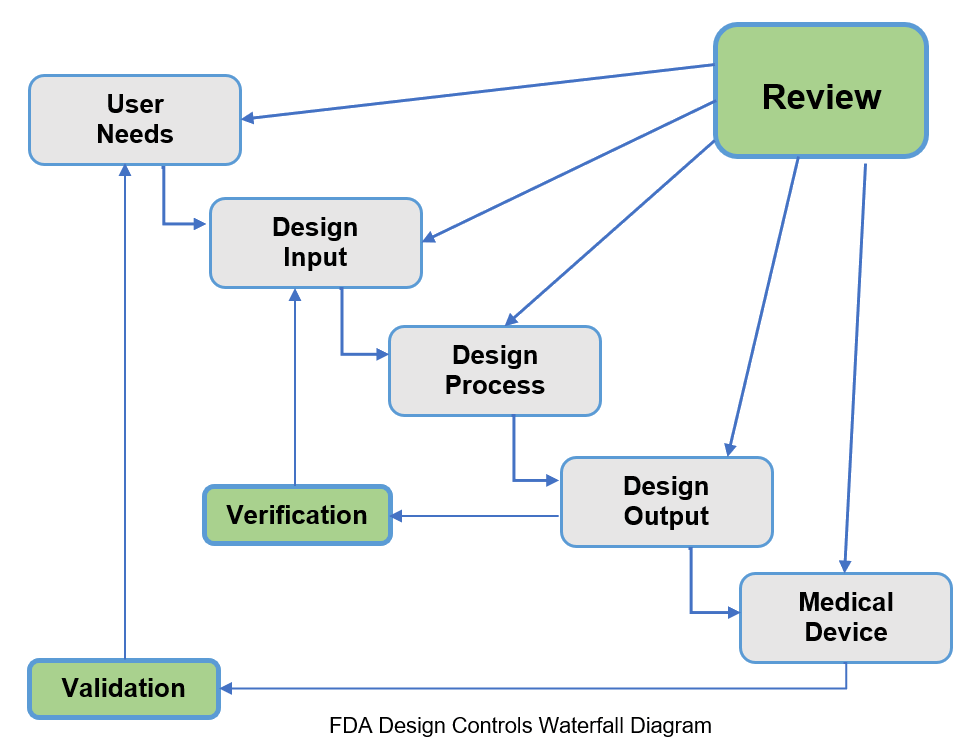
ISO and FDA refer to ?stages? and the FDA Design Controls waterfall diagram shows reviews for:
- User Needs
- Design Inputs
- Design Process
- Design Output
- Medical Device
I have found it common practice for the initial formal design review to be held after completion of the design inputs and to include a review of the user needs. Certainly for all the stages ad hoc meetings may be held to review specific areas of the design and unless there are major issue these are not often documented.
During the design process the number of reviews can depend on the complexity of the device and can be planned accordingly. Conduct as many as you need during the design process.
Design Outputs and how they meet the design inputs will need a formal design review. Design verification can be a separate review or included in a design process review. Design verification should be reviewed and prior to design validation. Before design transfer begins a formal review should be carried out and is often followed by another follow-up formal review some pre-selected time after the device has been in production.
Plan your design reviews far enough in advance to provide the invited participants time to prepare. Circulate an agenda and stick to it. Set a time limit of 60 to 90 minutes.
Conducting:
Design Reviews should be led by the project leader and include project team members and participants representing all functions concerned with the design stage being reviewed. Depending on the stage of the design and purpose of the review this could include reviewers from the following areas:
- Design Engineering
- Manufacturing Engineering
- Quality and Regulatory
- Manufacturing Operations
- Purchasing
- Marketing
Also need to include at least one independent reviewer not having direct responsibility for the design stage being reviewed, as well any specialists needed.
Purchasing and Marketing may not need to be in every review and for example Marketing should be part of reviewing design inputs and design outputs, and Purchasing should be involved for any outsourced or purchased component and materials reviews. Specialists who can be included as required may include microbiologist for sterilization reviews, packaging engineers, etc.
The purpose of the design review is not to just rubber stamp the design as is, but to obtain feedback in order to ensure the design of the device is safe, effective and will meet its intended purpose. Encourage the review attendees to respectively raise concerns and issues and make sure the design project leader knows how to accept the input in a constructive manner.
Another tip I like to remind design teams is to have as appropriate, any samples or prototypes that are available, and represent the design stage being reviewed. Nothing quite as effective as having a touch-and-feel sample to aid the design review process.
Some of the best design reviews I have participated in had all of these elements, plus each agenda item had a target time period allotted prior to the meeting to help keep things on track. Also, either the project leader, or a designated meeting facilitator, acted as a guide to help keep the participants on agenda and help prevent sidetracks from dragging things out.
Keep a documented checklist or record of your review as you go and this will help to complete the design review meeting minutes and documentation for follow up, and for your Device History File.
Follow up:
Not a regulatory requirement, but I have found it to be good practice, if once the project leader has documented the minutes of the meeting including any agreed upon follow up items, that prior to circulating the minutes, that they obtain approval signatures from the meeting participants. This is a good way to ensure that nothing was missed, and everyone is on board with the review and any planned actions.
Action items will very likely be identified during the design review and they need to be documented in the minutes of the review and followed up on and any design changes documented appropriately. Those actions should be part of the agenda for review at the next design review meeting.
Final thoughts:
Design Reviews are critical to the design and development process and with an open constructive team participation can play a significant role in the success of your medical device design. It?s really worth the effort to ensure all review participants understand before going into the reviews the importance of their full involvement and contributions.
Plan your reviews ahead of time and give at least one week?s notice and share the information to be reviewed.
Spend time on your documentation as it helps you manage the follow up items and of course the external auditors are going to be taking a detailed look at your design files including design reviews.
There is software available specifically designed for medical device quality systems including design and development activities that can make managing the control of your product throughout its lifecycle a lot easier. Fast-Track QMS Consultants can provide guidance on the selection of the right software to meet the needs of your medical device design and development process and manage your quality management system.
???????
