??????????A well designed and written Quality Manual should be easy to read and clearly correspond to the elements of the ISO 13485 standard elements as well as describe the companies quality management system. It serves as the foundation of the documented quality system and is a requirement of ISO 13485:2016 and most other quality management systems. The US FDA is currently an exception where it is not a documented requirement but expect this to change as they head towards adopting the ISO 13485 Standard.
In basic terms the Quality Manual can be considered as the user?s guide to your QMS and is a good reference for your management and employees. It is also where external auditors will start reviewing your QMS. It needs to be easy to read and cover the applicable requirements of ISO 13485:2016
One of the requirements of ISO 13485:2016 is that the scope of your quality management system be documented in the quality manual along with any justification for any exclusions. It is also a requirement that procedures for your quality management system be included in the manual or a reference to them which can be in the manual itself or in a supporting document, which in my experience is perhaps a better method.
The Quality Manual requirements to meet ISO 13485:2016 Section 4.2 Documentation requirements:
4.2.1. General
The quality management system documentation (see 4.2.4) shall include:
a) documented statements of a quality policy and quality objectives.
b) a quality manual
c) documented procedures and records required by this International Standard.
d) documents, including records, determined by the organization to be necessary to ensure the effective planning, operation, and control of its processes.
e) other documentation specified by applicable regulatory requirements.
4.2.2 Quality manual
The organization shall document a quality manual that includes:
a) the scope of the quality management system, including details of and justification for any exclusion or non-application.
b) the documented procedures for the quality management system, or reference to them.
c) a description of the interaction between the processes of the quality management system.
The quality manual shall outline the structure of the documentation used in the quality management system.
Scope of the quality management system:
It is a requirement that you document the scope of your QMS, including any exclusions in sections 6,7 and 8 for your company along with the justification for those exclusions.
One example could be if your company is manufacturing a disposable product then installation and maintenance is not applicable, and you could state:
???????????????????
XYZ Health Products designs and manufactures xyz and where installation and servicing activities are not applicable to non-reusable xyz products manufactured by XYZ Health Products.
General recommendations for writing your Quality Manual in the following areas
- Documentation of procedures
- Interaction of quality system processes
- Structure of the quality system documentation
- Other sections I would recommend for your Quality Manual
1. Documentation of procedures
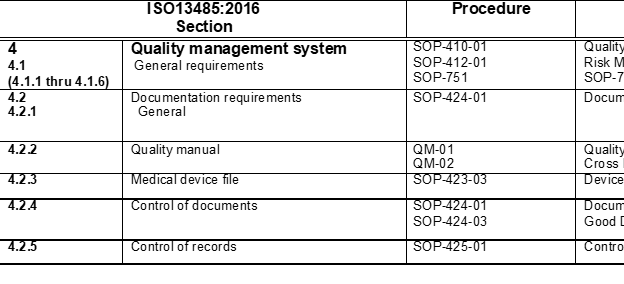
Most quality systems I have worked with or have reviewed have around 50 procedures +/- depending on the medical device, manufacturing processes and markets, but whatever the number of SOP?s they all need to be documented in the quality manual or referenced.
It?s most often not a good idea to document procedures in the quality manual as this would create a very bulky manual and difficult to manage, instead in my opinion, the best practice is to provide a brief top-level summary of the QMS process used under each element, and then use an appendix or quality manual supporting document that list all of the procedures and include a reference to the ISO 13485 element for each.
I have seen this referred to as a Cross Reference Addendum and see below for a sample of a section of this document:
2. Interaction of quality system processes
Different ways of showing this in the quality manual or can reference documents outside of the quality manual.
One of the most efficient ways I have seen for larger companies with many processes is to just list the major quality system processes in the manual itself then refer to external SIPOC flow diagrams attached to a QMS procedure.
The flow charts need to show inputs and outputs and the interaction between the processes.
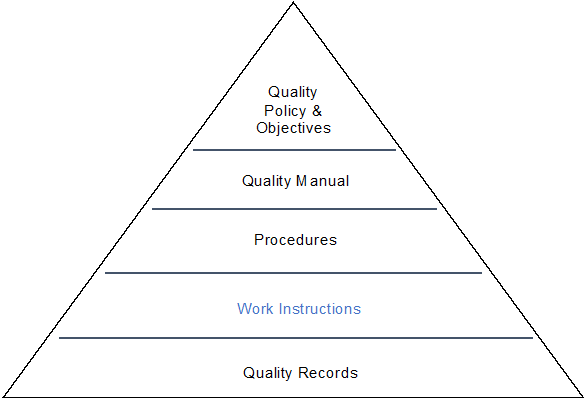
3. Structure of the quality system documentation:
The quality manual needs to show the structure of your quality management system and the simplest and most common way of showing this is in the form of a documentation pyramid as illustrated below:
You can determine the structure of your documentation and I have seen the quality manual
placed on top which is fine and of course if you don?t use Work Instructions in your quality system then you would not show that level.
4. Other sections I would recommend for your Quality Manual:
- Index: obvious yes, but good way to make navigating the manual easier
- Quality Policy: Quality Manual, great location to document your Quality Policy
- Applicable Standards and Regulations: list those applicable to your markets.
- Definitions: and abbreviations used in your manual
??????
- Cross Reference Addendum: as already mentioned it is more efficient to have QMS procedures referenced in a separate document rather that in your Quality Manual, although either way is ok as far as the standards go but listing in an attachment is I have found easier to navigate and find the appropriate procedure.
- Quality Plan: not strictly a requirement from most auditors, at least from my experience, but a good document to have for your QMS and can be a simple one-page plan for your QMS planned changes for the year.
- Process Flow Charts: this is to show how all your quality management processes interact with each other. Needs to show inputs and outputs for each process and needs to include all of your key processes including as appropriate, design and development, supplier approval, purchasing, manufacturing processes, and quality control steps.
- Organization Charts: from experience, best practice is to reference location in the Quality Manual and maybe appendix to a procedure. Also due to number of likely changes best to show only job positions and not name of person currently holding that position.